
NO&T IP Law Update
On May 27, 2025, in a patent infringement lawsuit brought by the patentee of a patented invention against two pharmaceutical companies manufacturing and selling generic versions of the patentee’s drug, the Intellectual Property High Court of Japan issued a judgment ordering the two generic drug companies to pay the patentee approximately JPY 21,762 million in total. The case is notable for several reasons including (i) the court’s interpretation of the term “active ingredient” in the claim and (ii) the court’s ruling on the scope of the patent during its extended term.
In this newsletter, we summarize the history of this case, explain the decision rendered by the Intellectual Property High Court, and provide a brief commentary on the decision.
Toray Industries, Inc. (“Toray”) is the patentee of Japanese Patent No. 3531170 (the “Patent”), titled “Antipruritic agent.” The filing date of the Patent is November 21, 1997.
On June 29, 2017, Toray filed an application for patent term extension of the Patent (Application No. 2017-700154, the “PTE Application”). Toray requested an extension for five years on the grounds that the marketing authorization (the “Marketing Authorization”), as generally described in Table 1, was required to implement the patented invention. As a result of the PTE Application, the term of the patent was deemed to have been extended pursuant to Article 67-2(5) of the Patent Act. The major developments in the history of the PTE Application are identified in Table 2.
Table 1: Details of the Marketing Authorization
Table 2: Major Developments in the History of the PTE Application
| Date | Event |
|---|---|
| June 29, 2017 | Toray files the PTE Application with the Japan Patent Office (the JPO) |
| March 5, 2018 | Issuance of the JPO examiner’s decision rejecting the PTE Application |
| June 1, 2018 | Toray’s submission of a request for review (i.e., appeal of the examiner’s decision) by the JPO’s Trial and Appeal Board (Case No. Appeal 2018-7539) |
| March 30, 2020 | Issuance of the decision dismissing the appeal (of the examiner’s decision) by the Trial and Appeal Board |
| March 25, 2021 | Issuance of Intellectual Property High Court’s Judgment rescinding the decision by the Trial and Appeal Board (Case No. Intellectual Property High Court, 2020 (Gyo-Ke) 10063) |
| July 9, 2021 | Issuance of the JPO’s decision granting the patent term extension (i.e., approval of the PTE Application) |
| August 11, 2021 | Registration of the patent term extension (the “PTE Registration”) |
| September 29, 2021 | Sawai Pharmaceutical Co., Ltd. files a request for invalidation of the PTE Registration (Case No. Invalidation 2021-800083) with the JPO |
| February 28, 2024 | Issuance of the decision dismissing the above invalidation request by the Trial and Appeal Board |
| May 27, 2025 | Issuance of the Intellectual Property High Court’s Judgment upholding the decision by the Trial and Appeal Board (Case No. Intellectual Property High Court, 2024 (Gyo-Ke) 10033) |
Claim 1 of the Patent reads as follows:
“An antipruritic agent comprising an opiate κ receptor agonist represented by the general formula (I) as an active ingredient:
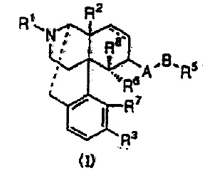
wherein […]”
The invention (the “Invention”) claimed in Claim 1 of the Patent can be structured by means of the following elements:

Sawai Pharmaceutical Co., Ltd. (“Sawai”) and Fuso Pharmaceutical Industries, Ltd. (“Fuso”) have been manufacturing and selling generic versions of Toray’s Drug (“Defendants’ Drugs”) since June 15, 2018.
In response to the perceived patent infringements by Sawai and Fuso, Toray filed a lawsuit against Sawai and Fuso seeking, among other relief, an injunction against the manufacture and sale of the Defendants’ Drugs and compensation for damages sustained as a consequence of the infringements, arguing that the Defendants’ Drugs satisfy all the elements of the Invention literally or under the doctrine of equivalents.
When comparing the Defendants’ Drugs with Toray’s Drug, the following points were not in dispute between the parties: (i) while the nalfurafine hydrochloride contained in Defendants’ Drugs does not fall under the description “an opiate κ receptor agonist represented by the general formula (I)” in Element A of the Invention, nalfurafine (free form) does fall under the Compound, and (ii) the Defendants’ Drugs fall under the description “an antipruritic agent” in Element B of the Invention.
On the other hand, with respect to the Defendants’ Drugs, whether nalfurafine hydrochloride or nalfurafine (free form) falls under the term “active ingredient” in Element A of the Invention was in dispute between the parties.
Toray argued that:
In response, Sawai and Fuso argued that:
The Tokyo District Court did not find that the Patent was infringed by Sawai and Fuso and dismissed Toray’s claims by ruling that the Defendants’ Drugs do not satisfy the elements of the Invention literally or under the doctrine of equivalents and thus do not fall within the technical scope of the Invention.
Regarding the main issue of whether the Defendants’ Drugs comprise nalfurafine (free form) which falls within the Compound, and falls under the term “active ingredient,” under Element A, the Court:
For such reason, the Court determined that, since the Defendants’ Drugs comprise nalfurafine hydrochloride as the drug substance, with respect to the Defendants’ Drugs, it is nalfurafine hydrochloride that falls under the description “active ingredient” in Element A, and thus, the Defendants’ Drugs do not satisfy Element A.
A patent term extension (PTE) (i.e., an extension of the term of a patent beyond the standard duration) may be granted when a marketing authorization is required to implement the patented medicinal invention (Articles 67(4) and 67-7(i) of the Patent Act).
The examiner of the Japan Patent Office (the “JPO”) decided to reject the PTE Application and Toray filed a request for review※2 to the Trial and Appeal Board (the “Board”) of the JPO.
On March 30, 2020, the Board dismissed the appeal (i.e., Toray’s request for review of the examiner’s decision rejecting the PTE Application).
The Board stated that, considering the prosecution history of the Patent, it can be understood that an antipruritic agent comprising “a pharmacologically acceptable acid addition salts of an opiate κ receptor agonist represented by the general formula (I),” such as nalfurafine hydrochloride, as an active ingredient, was included in the claim at the time of the Patent’s filing, but was excluded from the claim by the amendment of the claim dated July 16, 2001 (the “Amendment”).
In response to Toray’s argument that “active ingredient” in the Invention refers to the substance (nalfurafine) that exerts an effect in the body, the Board determined that (i) an “ingredient” generally refers to each substance constituting a mixture, (ii) in the case of pharmaceuticals, an “active ingredient” refers to the substance in the mixture that exhibits pharmacological effects, which is a matter of common technical knowledge, and (iii) therefore, the Invention does not include Toray’s Drug, which comprises nalfurafine hydrochloride as the active ingredient, and thus, it cannot be recognized that the Marketing Authorization was required to implement the Invention.
Toray appealed the Board’s decision for consideration by the Intellectual Property High Court.
In the appeal by Toray against the decision of the Board, the Intellectual Property High Court rescinded the Board’s decision.
The Intellectual Property High Court recognized the grounds for rescindment as follows: Ground 1 was an error in the factual findings by the Board regarding the active ingredient of Toray’s Drug, and Ground 2 was an error in the Board’s interpretation of the Invention.
Regarding Ground 1, the Court reviewed the contents of the Marketing Authorization and the package insert for Toray’s Drug, the common technical knowledge regarding the relationship between free forms and salts in the development of pharmaceuticals, the technical literature regarding the term “active ingredient,” and the recognition of the term “active ingredient” by pharmaceutical authorities. The Court stated that whether the Marketing Authorization was required to implement the Invention and the content of the Marketing Authorization should be determined substantively in light of the purpose of the patent term extension system. The Court further found that a person skilled in the art in the field of pharmaceuticals may consider not only addition salts blended in pharmaceuticals but also the free forms of substances as “active ingredients,” focusing on the activity that exerts the efficacy and effects that constitute the purpose of the pharmaceutical.
As a result, the Court determined that the active ingredient of Toray’s Drug is both the free form “nalfurafine” that was scrutinized as the ingredient that exerts the efficacy and effects during the review of the application for marketing authorization for Toray’s Drug, and the “nalfurafine hydrochloride,” which is contained in Toray’s Drug and is the form of the drug substance of Toray’s Drug. The Court thus concluded that the Board’s decision, which held that the Marketing Authorization was not required to implement the Invention, was erroneous. The Court made no determination on Ground 2, and no determination was made on the meaning of “active ingredient” in the Invention.
In addition to the PTE Application, three other applications※4 for patent term extensions were filed for the Patent, each of which was granted. Subsequently, requests for invalidation of the patent term extensions※5 were filed in relation to all such applications, and the JPO issued decisions invalidating the extensions. On appeal, the same panel of the Intellectual Property High Court as the one that handled the case mentioned in 2. above rescinded the decisions of the JPO for the same reason mentioned in 2. above.※6
Toray filed an appeal against the decision of the Tokyo District Court mentioned in III. above.
In the appeal proceeding, Toray alleged that it had been assigned the right to claim compensation for the damages suffered by Torii Pharmaceutical Co., Ltd. (“Torii”), the exclusive licensee and distributor of Toray’s Drug in Japan, and made an additional claim for damages based on such assigned right in addition to its original claim, in which Toray exercised its own right to claim compensation for damages suffered by Toray as the patentee. In addition, Toray withdrew its claim for an injunction against the manufacture and sale of the Defendants’ Drugs.
On appeal, the Intellectual Property High Court reversed the decision of the Tokyo District Court and ordered Sawai and Fuso to pay Toray damages of approximately JPY 14,290 million and JPY 7,472 million, respectively. The key determinations made by the Court are described below.
The court referred to the claims of the Patent and the description in the Specification, as well as relevant literature on “active ingredients,” and determined that:
Based on these findings, the Court stated that the Invention means an antipruritic agent where, regardless of whether it takes the form of an acid addition salt, a compound represented by general formula (I) is absorbed in the body and exerts pharmacological effects as an “active ingredient” based on its opiate κ receptor agonistic activity, and concluded that the Defendants’ Drugs satisfied the elements of the Invention and fell within the technical scope of the Invention.
As the filing date of the Patent is November 21, 1997, 20 years had already passed since the date of the filing of the Patent at the time of the commencement of the manufacture and sale of the Defendants’ Drugs.
The scope of the patent during the extended term is limited to the drug that was the subject of the marketing authorization on which the extension was based, and only for the use specified in that authorization (Article 68-2 of the Patent Act)※8. Thus, the Defendants’ Drugs infringe the Patent only if the manufacture and sale of the Defendants’ Drugs constitute the implementation of the Invention with respect to the drug that was the subject of the Marketing Authorization.
Sawai and Fuso alleged that they independently developed excipients for which they filed a patent application, rather than using well-known or conventional techniques, applied such excipients to the Defendants’ Drugs, and ensured the stability of nalfurafine without using sodium thiosulfate hydrate or other substances reported in academic papers or patent applications as effective stabilizers. Based on such facts, they argued (i) that the differences between the Defendants’ Drugs and Toray’s Drug should not be regarded as “minor or insignificant as a whole,” (ii) that the Defendants’ Drugs should not be considered to be substantially identical to Toray’s Drug, and therefore (iii) that the scope of the Patent during its extended term does not cover the Defendants’ Drugs.
The Court first referred to Debiopharm International SA v. Towa Pharmaceutical Co., Ltd. (Intellectual Property High Court, January 20, 2017)※9 and held that the effect of the patent during the extended term covers not only a “product” (drug) specified by the “ingredients, quantity, dosage, administration, indication, and usage” stipulated in the marketing authorization, but also those considered substantially identical as pharmaceuticals in light of the technical significance of the patented invention and the contents of the marketing authorization.
Furthermore, the Court pointed out that the technical feature of the Invention lies in the fact that the Invention is a medicinal use invention which uses a substance known at the time of the filing of the Patent as an active ingredient having an effect as an antipruritic agent, and stated that:
[i]n the case where the technical features and effects of [Toray’s Drug] and [the Defendants’ Drugs] are identical in that they are both antipruritic agents whose active ingredient is nalfurafine, and their specific dosage form as a pharmaceutical is identical, if some inactive ingredients are added or modified using techniques that were well-known and conventional at the time of the marketing authorization application, or if the differences between the inactive ingredients of [Toray’s Drug] and those of [the Defendants’ Drugs] do not affect the “indications and usage” of the drug and such differences are regarded as minor or insignificant as a whole, [the Defendants’ Drugs] should be deemed to be substantially identical to [Toray’s Drug] as pharmaceuticals.
Then the Court noted that the Invention is a medicinal use invention whose technical feature lies in that it provides a new medicinal use as an antipruritic agent based on the “κ receptor agonistic activity of compounds represented by general formula (I),” which was the unknown property, and that it does not specify any excipients to be contained in the antipruritic agent.
Furthermore, the Court noted that:
Furthermore, the Court noted that excipients are generally substances that do not exhibit pharmacological effects at the dosage of the drug, are harmless, and are added as substances not impairing the therapeutic effects of the active ingredient, and that in light of the description in the Specification and the development history of the Defendants’ Drugs, the excipients used in Toray’s Drug and the Defendants’ Drugs do not have any other technical significance.
Based on the above, the Court determined that because the technical features and effects of Toray’s Drug and the Defendants’ Drugs are identical in that they are both antipruritic agents whose active ingredient is nalfurafine, and their specific dosage form as a pharmaceutical is identical, in light of the significance of excipients, the differences in excipients between Toray’s Drug and the Defendants’ Drugs are regarded as minor or insignificant as a whole, and the Defendants’ Drugs are substantially identical to Toray’s Drug as pharmaceuticals.
With respect to damages, one of the issues was whether Torii, as the exclusive licensee and distributor of Toray’s Drug in Japan, had the right to make a claim for compensation for its damages.
The Court ruled on this point by reasoning that since Toray sells Toray’s Drug through Torii, and Torii is in a joint venture or similar relationship with Toray, Torii possesses legally protected interests equivalent to the benefit of having exclusive sales rights under the patent, and therefore, Torii has an independent right to claim compensation for its damages suffered from the patent infringements by Sawai and Fuso. Furthermore, the Court stated that the damages suffered by Toray and Torii constitute damages incurred by a joint venture engaged in the manufacture and sale of drugs utilizing the Patent, and that the difficulty in proving the amount of damages is similar to that faced by the patentee, and that therefore Article 102(1) of the Patent Act※10 may be analogously applied to calculate the damages suffered by Torii. Thus, the Court concluded that Toray, which had been assigned the right to claim damages from Torii, was thus entitled to claim compensation for damages equivalent to the lost profits under Article 102(1) of the Patent Act for each of Toray and Torii.
Based on this, the Court calculated the amount of damages for the Defendants’ Drugs that had been already sold pursuant to Article 102(1) of the Patent Act and calculated the amount of damages for the Defendants’ Drugs that had been already manufactured but not yet sold pursuant to Article 102(3) of the Patent Act※11.
Following the Intellectual Property High Court’s rescindment of the Board’s decision dismissing the appeal from the JPO examiner’s decision to reject the PTE Application mentioned above in Section IV, the PTE Registration was made on August 11, 2021.
In response, on September 29, 2021, Sawai filed a request for invalidation of the PTE Registration.※13 Sawai argued, among other points, that Toray’s Drug, whose active ingredient is nalfurafine hydrochloride, does not fall under the invention-specifying matters of the Invention, and therefore it cannot be recognized that the Marketing Authorization was required to implement the Invention. On February 28, 2024, the JPO issued a decision dismissing the request.
Sawai filed an appeal with the Intellectual Property High Court to overturn the JPO’s dismissal of its request. The case was heard by the same panel of the Intellectual Property High Court that rendered the decision mentioned above in Section VI.
At first, the Court referred to Takeda Pharmaceutical Co., Ltd. v. JPO (Supreme Court, April 28, 2011)※14 and Genentech, Inc. v. JPO (Supreme Court, November 17, 2015)※15 and in accordance with those decisions, stated that for a patent term extension to be granted, the subject of the marketing authorization that served as the basis for the extension application must implement, i.e. be within the technical scope of, the patented invention claimed in the claims of the patent for which an extension is sought. Furthermore, as stated in relation to the Court’s decision mentioned above in Section IV, the Court referred to the claims of the Patent and the description in the Specification, as well as relevant literature on “active ingredients,” and determined that:
Based on these findings, the Court stated that the Invention means an antipruritic agent where, regardless of whether it takes the form of an acid addition salt, a compound represented by general formula (I) is absorbed in the body and exerts pharmacological effects as an “active ingredient” based on its opiate κ receptor agonistic activity, and concluded that Sawai’s argument was groundless because Toray’s Drug satisfied the invention-specifying matters of the Invention and the Marketing Authorization was required to implement the Invention.
Sawai Pharmaceutical Co., Ltd. v. Toray Industries, Inc. (Intellectual Property High Court, May 27, 2025) dealt with the same issue as Toray Industries, Inc. v. JPO (Intellectual Property High Court, March 25, 2021) mentioned above in Section IV.2 did—whether the Marketing Authorization was required to implement the Invention. However, the framework of dealing with the issue in the former decision (2025) differs from that of the latter decision (2021). Specifically, in the latter decision (2021), the Court interpreted the meaning of “active ingredient” independently of the meaning of the “active ingredient” in the Invention, concluding (i) that the active ingredient of Toray’s Drug is the free form “nalfurafine” that was scrutinized as the ingredient that exerts the efficacy and effects during the review of the application for marketing authorization for Toray’s Drug, and the “nalfurafine hydrochloride,” which is contained in Toray’s Drug and is the form of the drug substance of Toray’s Drug, and (ii) that the Board’s decision, which held that the Marketing Authorization was not required to implement the Invention, was erroneous. On the other hand, in the former decision (2025), the Court interpreted the meaning of “active ingredient” in the Invention and determined that the Invention means an antipruritic agent where, regardless of whether it takes the form of an acid addition salt, a compound represented by general formula (I) is absorbed in the body and exerts pharmacological effects as an “active ingredient” based on its opiate κ receptor agonistic activity, concluding that Toray’s Drug satisfied the invention-specifying matters of the Invention and the Marketing Authorization was required to carry out the specific implementation of the Invention.
Toray Industries, Inc. v. Sawai Pharmaceutical Co., Ltd., et al. (Intellectual Property High Court, May 27, 2025) is one of the few court decisions to directly rule on the scope of the patent during its extended term as one of the central issues in a case where the court determines that, or the alleged infringer admits that, the allegedly infringing product falls within the technical scope of a patent for which a patent term extension was granted. This decision is highly notable because while it followed the general standard established in Debiopharm International SA v. Towa Pharmaceutical Co., Ltd. (Intellectual Property High Court, January 20, 2017), it did not focus exclusively on the four specific categories of drugs that may be considered “substantially identical” exemplified by Debiopharm; but rather it made detailed findings regarding the technical features and effects of the patented invention, the relationship between these features and both drugs, and the technical significance of the differing ingredients between Toray’s Drug and the Defendants’ Drugs in relation to the indications and usage of the drugs, to conclude that the differences in excipients between Toray’s Drug and the Defendants’ Drugs are regarded as minor or insignificant as a whole, and that the Defendants’ Drugs are substantially identical to Toray’s Drug as pharmaceuticals.
Sawai and Fuso have reportedly filed a final appeal and a petition for acceptance of final appeal against this decision. Whether the Supreme Court will take this case for review and, if so, what judgment it will render, are matters of interest. We will keep you informed.
*1
Case Nos. Tokyo District Court, 2018 (Wa) 38504, Tokyo District Court, 2018 (Wa) 38508
*2
Case No. Appeal 2018-7539
*3
Case No. Intellectual Property High Court, 2020 (Gyo-Ke) 10063
*4
Application Nos. 2015-700061, 2017-700309, 2017-700310
*5
Case Nos. Invalidation 2020-800002, 2020-800003, 2020-800004
*6
Case Nos. Intellectual Property High Court, 2020 (Gyo-Ke) 10098, Intellectual Property High Court, 2020 (Gyo-Ke) 10097, Intellectual Property High Court, 2020 (Gyo-Ke) 10096
*7
Case No. Intellectual Property High Court, 2021 (Ne) 10037
*8
For the background on the scope of the patent during its extended terms, please refer to NO&T IP Law Update No.13, “Tokyo District Court denies patent infringement claim in a case addressing the scope of a patent during its extended term (May 15, 2025)” (June 2025).
*9
Case No. Intellectual Property High Court, 2016 (Ne) 10046
*10
In short, under Article 102(1) of the Patent Act, the patentee can claim compensation for damages in the amount calculated by the profit that the patentee gains per patentee’s product multiplied by the number of products sold by the infringer.
*11
In short, under Article 102(3) of the Patent Act, the patentee can claim compensation for damages in the amount equivalent to the reasonable royalty that the patentee would receive under a patent license agreement for the infringed patent.
*12
Case No. Intellectual Property High Court, 2024 (Gyo-Ke) 10033
*13
Case No. Invalidation 2021-800083
*14
Case No. Supreme Court, 2009 (Gyo-Hi) 326, Minshu Vol. 65, No. 3, p. 1654
*15
Case No. Supreme Court, 2014 (Gyo-Hi) 356, Minshu Vol. 69, No. 7, p. 1912
This newsletter is given as general information for reference purposes only and therefore does not constitute our firm’s legal advice. Any opinion stated in this newsletter is a personal view of the author(s) and not our firm’s official view. For any specific matter or legal issue, please do not rely on this newsletter but make sure to consult a legal adviser. We would be delighted to answer your questions, if any.


(October 2025)
Kenji Tosaki


Claire Chong, Nozomi Kato (Co-author)


(September 2025)
Kenji Tosaki


(August 2025)
Oki Mori, Akiko Inoue (Co-author)


(October 2025)
Kenji Tosaki


(September 2025)
Kenji Tosaki


Kenji Tosaki, Ryo Tonomura (Co-author)


Kenji Tosaki, Takahito Hirayama (Co-author)


(October 2025)
Kenji Tosaki


(September 2025)
Kenji Tosaki


Kenji Tosaki, Ryo Tonomura (Co-author)


(June 2025)
Keiji Tonomura, Yukiko Konno, Minh Thi Cao Koike, Yoshiteru Matsuzaki (Co-author)


Kenji Tosaki, Ryo Tonomura (Co-author)


Kenji Tosaki, Takahito Hirayama (Co-author)


Kenji Tosaki, Nozomi Kato (Co-author)


(April 2025)
Kenji Utsumi, Kensuke Suzuki (Co-author)


Kenji Tosaki, Ryo Tonomura (Co-author)


(June 2025)
Shin Koike


Kenji Tosaki, Takahito Hirayama (Co-author)


(May 2025)
Shin Koike